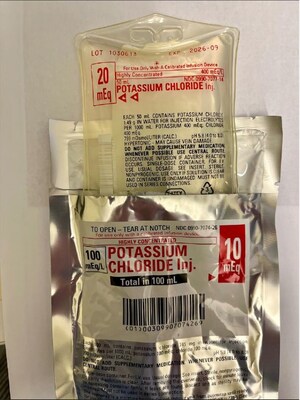
Otsuka ICU Medical LLC Issues Voluntary Nationwide Recall of 20 mEq Potassium Chloride Injection Due To Overwrap Mislabeled As 10 mEq Potassium Chloride Injection
PR Newswire
AUSTIN, Texas, Oct. 31, 2025
AUSTIN, Texas, Oct. 31, 2025 /PRNewswire/ -- Otsuka ICU Medical LLC is issuing a voluntary recall to the user level, for a MISLABELED lot of POTASSIUM CHLORIDE Inj. 20 mEq, NDC 0990-7077-14. The OVERWRAP label of lot 1030613, Expiration Date: 09-30-2026 may incorrectly identify the product as POTASSIUM CHLORIDE Inj. 10 mEq with NDC 0990-7074-26. Otsuka ICU Medical LLC has identified this discrepancy due to a manufacturing issue. The dosage is correctly printed on the labeling affixed to the product bag which is not visible when the 10 mEq OVERWRAP is in place. This notification details the issue and the required steps for you to perform.
If the incorrect dosage on the 10 mEq overwrap is used instead of the correct 20mEq dosage printed on the product, an overdose of potassium chloride is possible. Overdose of potassium chloride can lead to hyperkalemia. Hazards of severe hyperkalemia after large intravenous overdoses causes neuromuscular dysfunction including muscle weakness, ascending paralysis, listlessness, vertigo, mental confusion, hypotension, cardiac dysrhythmias, or death from cardiac arrest. Premature infants, patients on chronic parenteral nutrition, patients who have a history of cardiac arrythmias, patients with chronic renal insufficiency, patients who have acute renal failure, patients on potassium-sparing diuretics—all are at risk for adverse and potentially fatal outcomes. Otsuka ICU Medical LLC has not received reports of adverse events associated with this issue to date.
INDICATIONS AND USAGE:
Potassium Chloride Injection 20 mEq and 10 mEq, is indicated in the treatment of potassium deficiency states, when oral replacement is not feasible.
THIS HIGHLY CONCENTRATED, READY-TO-USE POTASSIUM CHLORIDE INJECTION IS INTENDED FOR THE MAINTENANCE OF SERUM K+ LEVELS AND FOR POTASSIUM SUPPLEMENTATION IN FLUID RESTRICTED PATIENTS WHO CANNOT ACCOMMODATE ADDITIONAL VOLUMES OF FLUID ASSOCIATED WITH POTASSIUM SOLUTIONS OF LOWER CONCENTRATION. TO AVOID POTASSIUM INTOXICATION, DO NOT INFUSE THESE SOLUTIONS RAPIDLY.
When using these products, these patients should be on continuous cardiac monitoring and frequent testing for serum potassium concentration and acid-base balance.
The affected product lot was manufactured on 15 April 2025 and distributed in the United States between 23 May 2025 through 26 August 2025. The affected product lot (Located on the top left of the product bag or the case label is:
NDC Number | List Number | Product Description | Lot Number | Expiration Date | Configuration |
0990-7077-14 | 070770452 | POTASSIUM CHLORIDE Inj. 20 mEq | 1030613 | 30 September 2026 | 50mL in Flexible Container |
0990-7074-26 | 070740452 | POTASSIUM CHLORIDE Inj. 10 mEq | N/A | N/A | 100mL in Flexible Container |
DESCRIPTION OF CASES BEING RECALLED:
NDC Number | Barcode Number | Lot Number | Expiration Date | Configuration |
0990-7077-14 | (01)20309907077141 | 1030613 | 30 September 2026 | 24/case |
Otsuka ICU Medical LLC is notifying its customers, including distributors, of this recall by letter and is arranging for the return of all recalled products. All Customers, including distributors, that have product that is being recalled should stop use/further distribution, as applicable, and return to place of purchase.
To return affected product or if you require assistance, please contact Sedgwick at 1-888-566-2363 (M-F, 8am to 5pm ET) to obtain a return label.
For further inquiries, please contact Otsuka ICU Medical LLC using the information provided below.
Otsuka ICU Medical LLC Contact | Contact Information | Areas of Support |
Global Complaint Management |
1-(866)-216-8806 | To report product complaints |
Drug Safety | 1-844-654-7780 or | To report adverse events for IV Solutions & Drugs |
Medical Information | 1-800-241-4002, option 6 or medinfo_us@icumed.com | Medical inquiries |
Customer Care |
1-(800)-258-5361 | Product Credit |
Field Action Processing | Questions about this action and response forms |
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
The U.S. Food and Drug Administration (FDA) has been notified of this action.
About Otsuka ICU Medical LLC
Otsuka ICU Medical LLC brings together global manufacturing strength, continuous innovation and a commitment to quality to deliver critical IV solutions for healthcare providers. With a broad portfolio of injectable, irrigation and parenteral nutrition products, the company is focused on building a resilient supply chain and advancing smarter, stronger patient care. Learn more at www.otsukaicumed.com.
Contact:
Consumers
ICU Medical, Inc.
1-844-654-7780
Media Contact:
Harrison Richards
ICU Medical, Inc.
949-366-4261
Harrison.Richards@icumed.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/otsuka-icu-medical-llc-issues-voluntary-nationwide-recall-of-20-meq-potassium-chloride-injection-due-to-overwrap-mislabeled-as-10-meq-potassium-chloride-injection-302601384.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/otsuka-icu-medical-llc-issues-voluntary-nationwide-recall-of-20-meq-potassium-chloride-injection-due-to-overwrap-mislabeled-as-10-meq-potassium-chloride-injection-302601384.html
SOURCE Otsuka ICU Medical LLC